Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...

Chiera, N. M.*; Sato, Tetsuya; Eichler, R.*; Tomitsuka, Tomohiro; Asai, Masato; Adachi, Sadia*; Dressler, R.*; Hirose, Kentaro; Inoue, Hiroki*; Ito, Yuta; et al.
Angewandte Chemie; International Edition, 60(33), p.17871 - 17874, 2021/08
Times Cited Count:2 Percentile:14.88(Chemistry, Multidisciplinary)The formation and the chemical characterization of single atoms of dubnium (Db, element 105), in the form of its volatile oxychloride, was investigated using the on-line gas phase chromatography technique, in the temperature range 350 - 600  C. Under the exact same chemical conditions, comparative studies with the lighter homologs of group-5 in the Periodic Table clearly indicate the volatility sequence being NbOCl
C. Under the exact same chemical conditions, comparative studies with the lighter homologs of group-5 in the Periodic Table clearly indicate the volatility sequence being NbOCl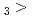 TaOCl
TaOCl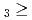 DbOCl
DbOCl . From the obtained experimental results, thermochemical data for DbOCl
. From the obtained experimental results, thermochemical data for DbOCl were derived. The present study delivers reliable experimental information for theoretical calculations on the chemical properties of transactinides.
were derived. The present study delivers reliable experimental information for theoretical calculations on the chemical properties of transactinides.
Chiera, N. M.; Sato, Tetsuya; Tomitsuka, Tomohiro; Asai, Masato; Ito, Yuta; Shirai, Kaori*; Suzuki, Hayato; Tokoi, Katsuyuki; Toyoshima, Atsushi; Tsukada, Kazuaki; et al.
Journal of Radioanalytical and Nuclear Chemistry, 320(3), p.633 - 642, 2019/06
Times Cited Count:1 Percentile:11.15(Chemistry, Analytical)An isothermal gas-chromatographic (IGC) device has been developed and tested for on-line gas phase studies of volatile oxychlorides of short-lived group-5 transition metals. Radioisotopes of niobium and tantalum, produced in nuclear fusion evaporation reactions, are directly flushed into the IGC setup by an inert gas-jet. Oxychloride compounds are formed by the addition of SOCl and O
and O . Parameters influencing the formation and transport of NbOCl
. Parameters influencing the formation and transport of NbOCl and TaOCl
and TaOCl are investigated. For nuclides with half-lives (
are investigated. For nuclides with half-lives (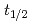 ) of about 30 s, an overall efficiency of 7% is obtained, rendering the IGC setup suitable for the chemical exploration of
) of about 30 s, an overall efficiency of 7% is obtained, rendering the IGC setup suitable for the chemical exploration of  Db(
Db(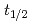 = 34s).
= 34s).
Chiera, N. M.; Sato, Tetsuya; Tomitsuka, Tomohiro; Asai, Masato; Suzuki, Hayato*; Tokoi, Katsuyuki; Toyoshima, Atsushi; Tsukada, Kazuaki; Nagame, Yuichiro
Inorganica Chimica Acta, 486, p.361 - 366, 2019/02
Times Cited Count:3 Percentile:18.6(Chemistry, Inorganic & Nuclear)The formation of NbOCl and TaOCl
and TaOCl and their adsorption behavior on quartz surfaces was explored by applying an isothermal gas-chromatographic method. Trace amounts of short-lived Nb and Ta isotopes were used. Adsorption enthalpy values (
and their adsorption behavior on quartz surfaces was explored by applying an isothermal gas-chromatographic method. Trace amounts of short-lived Nb and Ta isotopes were used. Adsorption enthalpy values ( ) at zero surface coverage of -
) at zero surface coverage of - (NbOCl
(NbOCl ) = 102
) = 102  4 kJ/mol and -
4 kJ/mol and - (TaOCl
(TaOCl ) = 128
) = 128  5 kJ/mol were determined by analyzing the chromatographic behavior of the Nb andTa complexes with a Monte-Carlo simulation method based on an adsorption-desorption kinetic model.By applying an empirical correlation, the experimental
5 kJ/mol were determined by analyzing the chromatographic behavior of the Nb andTa complexes with a Monte-Carlo simulation method based on an adsorption-desorption kinetic model.By applying an empirical correlation, the experimental  values were successively related to the macroscopic standard sublimation enthalpy,
values were successively related to the macroscopic standard sublimation enthalpy, 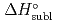 , as a measure of the volatility of each substance. The inferred sublimation enthalpies are in agreement with tabulated thermochemical values. Thus, the linear empirical correlation between
, as a measure of the volatility of each substance. The inferred sublimation enthalpies are in agreement with tabulated thermochemical values. Thus, the linear empirical correlation between  and
and 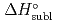 for metal-oxychlorides was updated with the inclusion of the present data. According to the predicted
for metal-oxychlorides was updated with the inclusion of the present data. According to the predicted 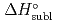 (DbOCl
(DbOCl ), a
), a  (DbOCl
(DbOCl ) value of 135
) value of 135  2 kJ/mol was extrapolated. The future accomplishment of comparative studies with DbOCl
2 kJ/mol was extrapolated. The future accomplishment of comparative studies with DbOCl under the same experimental conditions will provide valuable information on the volatility trend in Group-5 elements, together with an indication on the magnitude of relativistic effects on the electronic structure of dubnium.
under the same experimental conditions will provide valuable information on the volatility trend in Group-5 elements, together with an indication on the magnitude of relativistic effects on the electronic structure of dubnium.
 Nb and
Nb and  Ta for chemical studies of element 105, Db, using the GARIS gas-jet system
Ta for chemical studies of element 105, Db, using the GARIS gas-jet systemHuang, M.*; Haba, Hiromitsu*; Murakami, Masashi*; Asai, Masato; Kaji, Daiya*; Kanaya, Jumpei*; Kasamatsu, Yoshitaka*; Kikunaga, Hidetoshi*; Kikutani, Yuki*; Komori, Yukiko*; et al.
Journal of Radioanalytical and Nuclear Chemistry, 304(2), p.845 - 849, 2015/05
Times Cited Count:3 Percentile:25.85(Chemistry, Analytical)A technique to utilize radioisotopes of Nb and Ta was developed for chemical studies of element 105, Db, by coupling a gas-jet transport system to the RIKEN gas-filled recoil ion separator (GARIS). The short-lived  Nb and
Nb and 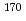 Ta were produced with nuclear reactions using a
Ta were produced with nuclear reactions using a  F beam whose energy was the same as that to produce
F beam whose energy was the same as that to produce  Db. Then, they were separated with GARIS and extracted to a chemistry laboratory with the gas-jet transport system. By changing only magnetic field of GARIS and inserting an energy degrader and a shutter for recoil ions, we could deliver the
Db. Then, they were separated with GARIS and extracted to a chemistry laboratory with the gas-jet transport system. By changing only magnetic field of GARIS and inserting an energy degrader and a shutter for recoil ions, we could deliver the  Nb and
Nb and 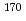 Ta to a chemistry device for
Ta to a chemistry device for  Db without changing other experimental conditions.
Db without changing other experimental conditions.
Chiera, N. M.; Sato, Tetsuya; Asai, Masato; Suzuki, Hayato*; Tokoi, Katsuyuki; Tomitsuka, Tomohiro; Toyoshima, Atsushi; Tsukada, Kazuaki; Nagame, Yuichiro
no journal, ,
 solution for understanding chemical species of Db
solution for understanding chemical species of DbAdachi, Sadia*; Sueki, Keisuke*; Toyoshima, Atsushi*; Tsukada, Kazuaki; Haba, Hiromitsu*; Komori, Yukiko*; Yokokita, Takuya*; Mori, Taiki*
no journal, ,
Anion exchange behavior of group-5 elements, Nb, Ta, and their pseudo homologue Pa in HF and HF/HNO solution was investigated to understand chemical properties of Db. Adsorption behavior of Db is clearly different from that of Ta and is similar to those of Nb and Pa. In the present study, anion exchange experiments of Nb, Ta and Pa were carried out with HF/HNO
solution was investigated to understand chemical properties of Db. Adsorption behavior of Db is clearly different from that of Ta and is similar to those of Nb and Pa. In the present study, anion exchange experiments of Nb, Ta and Pa were carried out with HF/HNO aqueous solution as basic experiments for estimating the species of Db fluoride complex. Adsorption behavior was investigated by changing the fluoride ion concentration for each HNO
aqueous solution as basic experiments for estimating the species of Db fluoride complex. Adsorption behavior was investigated by changing the fluoride ion concentration for each HNO concentration. Based on the observed distribution coefficients of these elements, we discuss the formation reaction of the anionic fluoride complex and chemical species of Db.
concentration. Based on the observed distribution coefficients of these elements, we discuss the formation reaction of the anionic fluoride complex and chemical species of Db.
 solution
solutionKato, Mizuho*; Adachi, Sadia*; Toyoshima, Atsushi*; Tsukada, Kazuaki; Asai, Masato; Haba, Hiromitsu*; Yokokita, Takuya*; Komori, Yukiko*; Shigekawa, Yudai*; Sueki, Keisuke*
no journal, ,
A few studies have been so far performed on aqueous chemistry of Db, the group-5 superheavy element, by anion-exchange experiments in HF and HF/HNO mixture solutions. Although the distribution coefficient (Kd) of Db was shown to trend to Ta
mixture solutions. Although the distribution coefficient (Kd) of Db was shown to trend to Ta  Nb
Nb  Db
Db  Pa in the group-5 homologs and pseudo-homolog elements, its chemical species has not been clarified. In this work, for identification of fluoride species of Db, we performed anion-exchange experiments of Nb, Ta, and Db in HF/1.0 M HNO
Pa in the group-5 homologs and pseudo-homolog elements, its chemical species has not been clarified. In this work, for identification of fluoride species of Db, we performed anion-exchange experiments of Nb, Ta, and Db in HF/1.0 M HNO mixture solutions by using an automated rapid chemistry apparatus, ARCA.
mixture solutions by using an automated rapid chemistry apparatus, ARCA.
Sato, Tetsuya; Chiera, N. M.*; Tomitsuka, Tomohiro; Tokoi, Katsuyuki*; Suzuki, Hayato*; Ito, Yuta; Asai, Masato; Shirai, Kaori*; Inoue, Hiroki*; Adachi, Sadia*; et al.
no journal, ,
The influence of strong relativistic effects on chemical properties has been interesting in the superheavy element region. Their chemical properties, however, have not been investigated sufficiently because of experimental difficulties owing to their low production rates and short half-lives. In order to elucidate the chemical properties of dubnium (Db, Z = 105), we have conducted on-line isothermal gas chromatographic experiments of oxychloride of group-5 elements. We confirmed the formation of volatile oxychlorides of Db and its lighter homologs Nb and Ta by using  Db (half-life,
Db (half-life, 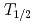 = 33.8 s),
= 33.8 s),  Nb (
Nb (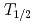 = 14.5 min.), and
= 14.5 min.), and 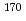 Ta (
Ta (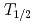 = 6.76 min.), respectively. We successfully determined the adsorption enthalpies of the oxychlorides of each element on the quartz surface from their isothermal gas chromatographic behavior. The obtained volatility sequence of the group-5 elements is found to be Nb
= 6.76 min.), respectively. We successfully determined the adsorption enthalpies of the oxychlorides of each element on the quartz surface from their isothermal gas chromatographic behavior. The obtained volatility sequence of the group-5 elements is found to be Nb  Ta
Ta  Db.
Db.